The pursuit of advanced battery technologies has never been more critical, particularly in light of the growing demand for efficient energy storage systems in electric vehicles and portable electronics. As researchers strive to develop batteries that can store greater amounts of energy, charge quickly, and last longer, much of the focus lies on optimizing cathode materials. In this context, layered lithium-rich transition metal oxides have emerged as a candidate that may significantly enhance battery performance. However, while these materials offer promising advantages, they also come with intrinsic challenges that greatly affect their application.
Layered lithium-rich transition metal oxides are gaining attention due to their unique structural properties, which allow lithium ions to move freely between layers during the charging and discharging processes. This layered configuration enhances the potential energy density of batteries, making them more effective for energy-intensive applications. The composition of these materials typically includes lithium, along with transition metals such as manganese, cobalt, or nickel, in conjunction with oxygen. These elements contribute to the critical electrochemical reactions that occur within batteries, specifically the redox processes that facilitate energy production.
However, a significant drawback is that many of these cathodes suffer from issues related to stability and degradation. Research has indicated that although they can deliver high energy density, they often experience rapid performance decline over time. This deterioration limits their potential for widespread commercial use, necessitating a deeper investigation into the mechanisms underlying their failure.
Recent Advances in Understanding Degradation Mechanisms
To address the limitations of layered lithium-rich cathodes, a cohort of researchers from Sichuan University and the Southern University of Science and Technology in China, along with international collaborators, embarked on a comprehensive study aimed at elucidating the factors leading to the degradation of these materials. Their findings, published in *Nature Nanotechnology*, shed light on the intricate and nuanced processes through which these cathodes lose functionality.
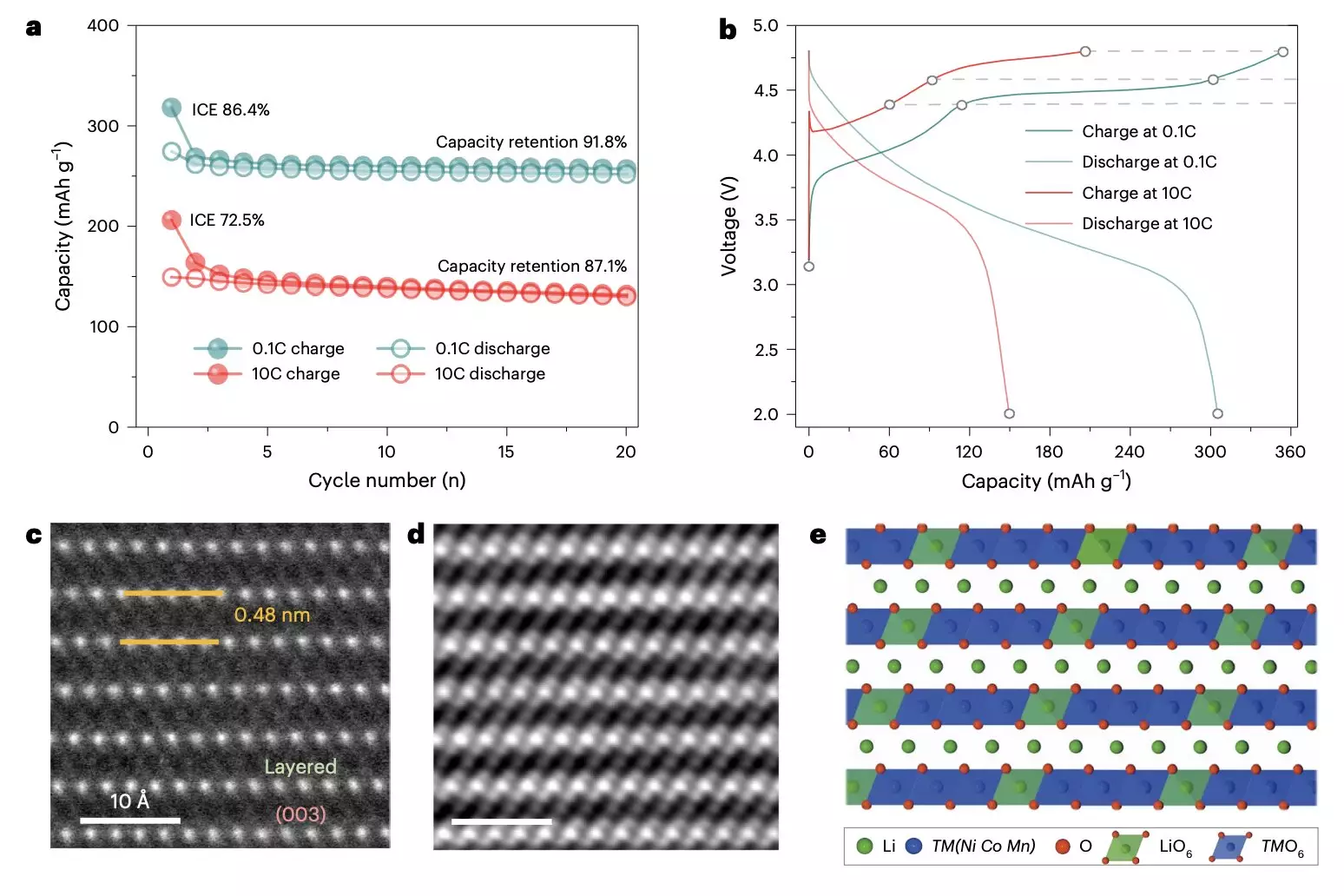
Utilizing innovative imaging techniques, including energy-resolved transmission X-ray microscopy (TXM), the researchers examined the structural and chemical transformations occurring at both nanoscale and microscale levels during operation. Insights gained from these observations revealed several key factors that contribute to the observed deterioration, including the formation of oxygen defects and internal distortions at varying charging rates. These defects can initiate a cascade of reactions that progressively undermine the integrity of the cathode material.
The Role of Electrochemical Activation
The study highlighted that slow electrochemical activation leads to significant challenges, including the emergence of nanovoids within the material. During rapid charge and discharge cycles, ultrafast (de)intercalation of lithium ions disrupts the stability of the lattice structure, resulting in displacement of the transition metal ions and variations in lithium occupation at their respective sites. These structural anomalies not only reduce the initial efficiency of the batteries but also contribute to ongoing mechanical stress that results in cracking and expansion over subsequent charging cycles.
Understanding these mechanisms is crucial for developing better battery technologies. By identifying the specific pathways through which structural degradation unfolds, researchers can formulate targeted approaches to mitigate these failures. Such improvements could greatly enhance the viability of layered lithium-rich transition metal oxides for next-generation battery applications.
The findings from this research have profound implications for the future of battery technology. As the demand for sustainable energy solutions continues to rise, the ability to improve the performance and lifespan of rechargeable batteries becomes increasingly vital. By developing strategies that address the degradation issues associated with layered lithium-rich metal oxides, researchers may pave the way for safer, more efficient energy storage systems.
While layered lithium-rich transition metal oxides represent a promising avenue for improving battery performance, challenges associated with their long-term stability must be overcome. Ongoing research in this field will not only unlock new insights into the fundamental behavior of these materials but also catalyze the development of next-generation batteries that could revolutionize energy storage and power supply across various sectors.