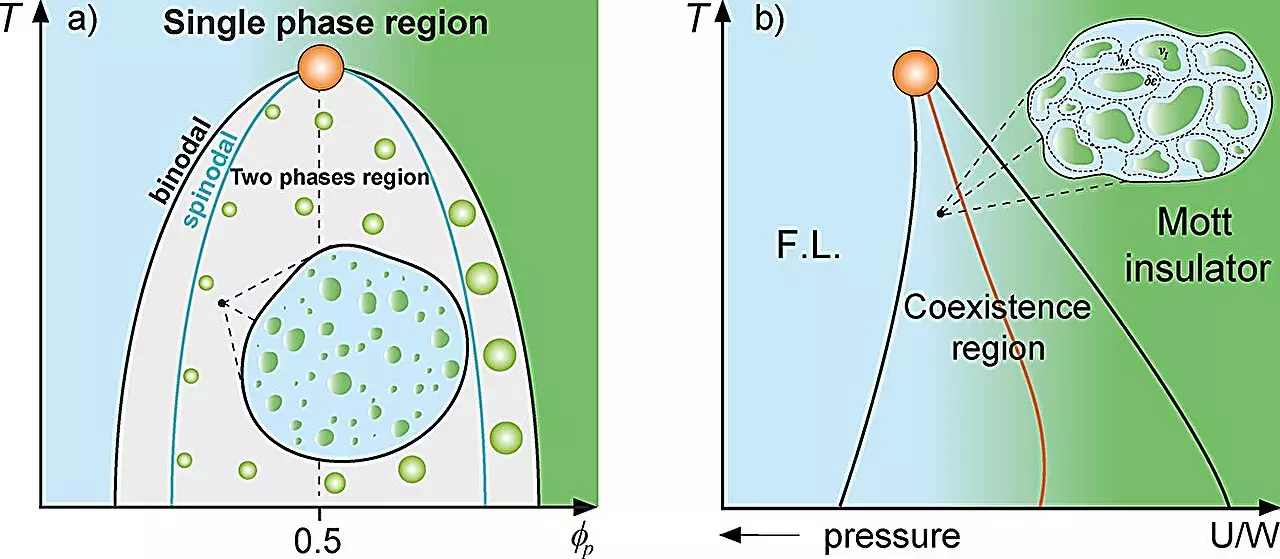
In the realm of physics, the study of material interactions often involves examining systems composed of varying components. Classical mixture theory provides a framework for modeling these systems by focusing on the distribution and interactions of each constituent. Diverse phenomena, such as the behavior of supercooled water’s distinct phases or the peculiar properties observed in the Mott metal-insulator transition, can be explored through this theoretical lens. Recent research spearheaded by scientists at São Paulo State University (UNESP) in Brazil has applied this theoretical framework to a biological context, specifically investigating how proteins interact within cellular environments. This innovative approach has yielded intriguing insights into protein compartmentalization, likening it metaphorically to known physical phenomena.
The research team, led by professor Mariano de Souza and Ph.D. candidate Lucas Squillante, explored the dynamics of protein droplets within cells, employing concepts drawn from condensed matter physics. Just as the magnetic Griffiths phase describes emergence dynamics within magnetized substrates, the researchers proposed a cellular analog characterized by protein droplets acting as rare regions. This study, published in the journal Heliyon, illustrates how applying principles from a physical science can enhance our understanding of biological processes.
The essence of this Griffiths-like cellular phase lies in the behavior of protein droplets that form through liquid-liquid phase separation—an event triggered when protein concentrations reach a critical threshold. This phase separation is not merely a trivial phenomenon; it plays a pivotal role in cellular organization and potentially in the origins of life itself. Professor Souza posits that the concepts derived from classical physics provide a fresh perspective on cellular dynamics and evolution.
Utilizing established thermodynamic frameworks, including the Grüneisen parameter and the Flory-Huggins model, the researchers showed that the cellular environment displays dramatically altered dynamics close to the binodal line—a demarcation of phase separation. The implications of their findings point to how the reduction of dynamic motion within these protein-rich droplets may lead to enhanced stability and efficiency in cellular processes.
By drawing parallels between the protein dynamics observed within cells and the broader principles of magnetism, the researchers make a compelling case that not only do these systems share structural interdependencies, but they also diverge dynamically. For instance, just as the presence of rare magnetic regions can significantly alter the properties of a material, the formation of protein droplets can influence gene expression and overall cellular function through reduced stochastic fluctuations.
One of the most striking aspects of this research is its potential connection to biogenesis, particularly through the lens of Aleksandr Oparin’s hypothesis on the origins of life. The study suggests that coacervates—aggregates of organic molecules—exhibited slow dynamics that were crucial for their survival and evolutionary potential in primordial environments. This perspective not only postulates a historical foundation for life on Earth but also invites further inquiry into how modern cellular machinery evolved from these fundamental processes.
Chirality, the property whereby an object cannot be superimposed upon its mirror image, further enriches this narrative. The predominance of homochirality, particularly in molecule formation within biological systems, plays a crucial role. The research underscores the need for a uniform chiral environment, which may have been essential for the development of complex life forms.
The study also casts light on the relationship between liquid-liquid phase separation and various diseases, an area of significant interest within medical research. From neurodegenerative diseases to cancer, the compartmentalization of proteins can affect cellular behavior in diverse ways. For instance, the research postulates that aberrant protein compartmentalization, especially related to diseases like cancer or cataracts, highlights the dual nature of these dynamics: while some formations can be beneficial, others may lead to detrimental mutations.
The interdisciplinary nature of this study exemplifies the importance of blending knowledge across fields—from physical science to biology—to generate comprehensive insights into complex phenomena. Through collaboration, researchers not only enhance our understanding of fundamental scientific principles but also create frameworks that can lead to innovative therapeutic interventions.
In sum, the investigation into Griffiths-like cellular phases offers a novel lens through which to view protein behavior in biological systems. As we delve deeper into these complex interactions, the intersection of physics and biology may uncover new avenues for understanding life at a molecular level. The implications of these findings extend beyond academia, paving the way for transformative possibilities in medical science and biotechnology. Embracing interdisciplinary research is not just important; it is essential for unraveling the intricacies of both our physical and biological worlds.