Recent advancements in the realm of neuroscience illustrate a growing trend towards non-invasive treatment methods for neurological disorders. One such technique gaining prominence is transcranial focused ultrasound (tFUS), which utilizes high-frequency sound waves to stimulate specific parts of the brain without the need for surgical intervention. This innovative approach not only opens new avenues for tackling drug-resistant epilepsy but also holds promise for conditions characterized by recurrent tremors, marking a significant leap in medical technology aimed at treating complex neurological conditions.
A collaborative effort involving researchers from Sungkyunkwan University (SKKU), the Institute for Basic Science (IBS), and the Korea Institute of Science and Technology (KIST) has birthed a groundbreaking sensor that enhances the efficacy of tFUS. This sensor has recently been showcased in a study published in *Nature Electronics*, highlighting its unique ability to adapt its shape and maintain close adherence to the complex architecture of cortical surfaces. By enabling accurate neural signal recordings and localized stimulation via low-intensity ultrasound waves, the sensor could revolutionize traditional methodologies for understanding and treating brain-related disorders.
Addressing Limitations of Previous Technologies
The journey towards an ideal brain sensor has been fraught with challenges, particularly regarding the accurate measurement of neural signals. Previous iterations of brain sensors displayed significant limitations, especially in achieving a reliable fit on uneven brain surfaces, such as regions exhibiting severe curvature. Donghee Son, the supervising author of the study, articulated this hurdle succinctly, stating that past sensors struggled to comply with the intricate folds of the brain. These complications hindered the comprehensive analysis of brain activity, making it difficult to diagnose and evaluate neurological conditions accurately.
Further investigation revealed that previous sensors developed by notable researchers John A. Rogers and Dae-Hyeong Kim had made strides in addressing some of these challenges, primarily by reducing their thickness. However, these sensors could not provide robust adhesion in regions with more pronounced curvatures, leading to potential slippage and inaccuracies when measuring brain signals. Such issues have limited their viability in clinical environments, where consistent and precise data collection is paramount for effective diagnosis and treatment.
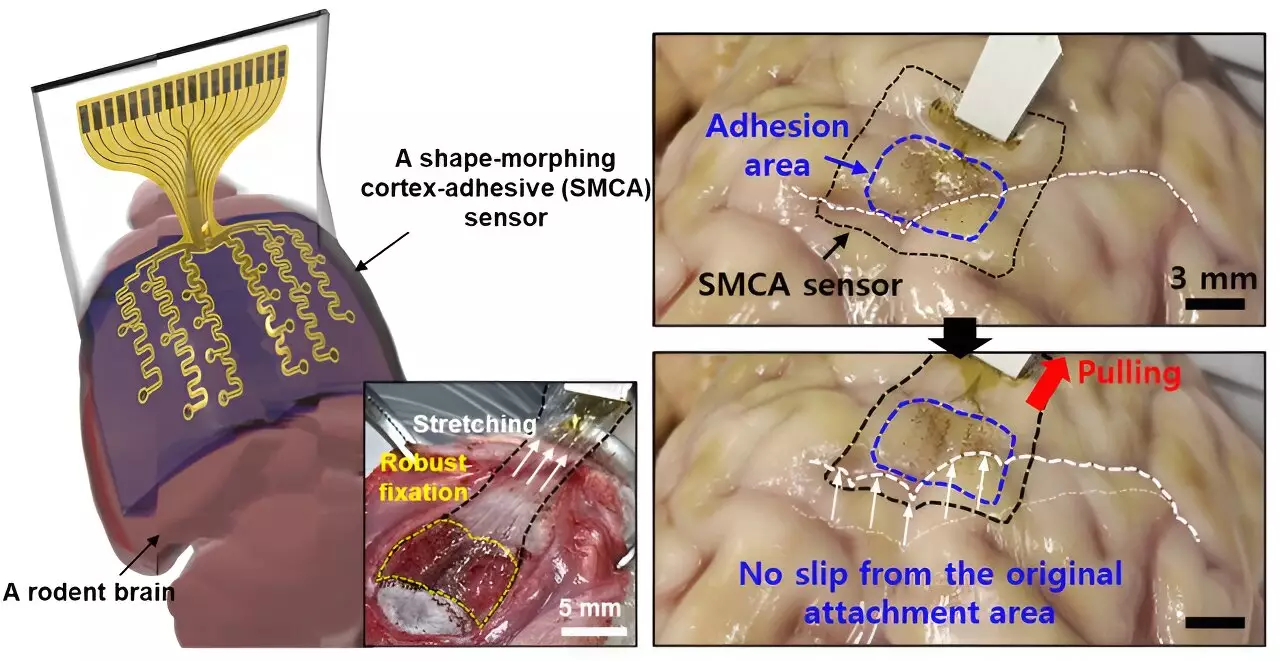
In response to these challenges, Son and his team engineered a novel sensor that rectifies the limitations of its predecessors. Dubbed the ECoG sensor, this innovative technology offers exceptional adherence to the brain’s surface, combining strong attachment with the ability to conform tightly to highly contoured regions. Son conveyed the importance of this development, emphasizing that it facilitates long-term measurements of brain activity while mitigating noise from external movements, thus significantly enhancing the capabilities of epilepsy treatments utilizing low-intensity focused ultrasound (LIFU).
This new sensor design employs a layered structure composed of a hydrogel-based layer, a self-healing polymer layer, and a stretchable layer integrated with gold electrodes. Each layer plays a crucial role in ensuring the sensor can securely attach to brain tissue, while also adapting to the diverse shapes presented by the brain’s surface. Once applied, the hydrogel initiates a robust attachment process, while the self-healing polymer actively adjusts to enhance contact over time, thereby minimizing any voids that could lead to inaccuracies in measurements.
The technological innovation behind the ECoG sensor may also pave the way for personalized treatments in neurology, particularly for conditions like epilepsy. Conventional sensors have struggled with real-time monitoring due to noise created by ultrasound vibrations, posing significant barriers to tailoring individualized treatment plans. However, the ECoG sensor significantly reduces noise levels, thus enabling healthcare providers to monitor brain waves accurately while simultaneously applying targeted ultrasound stimulation.
As researchers delve deeper into crafting personalized therapeutic strategies, the ECoG sensor stands out as a critical tool that can adapt to patient-specific needs and conditions. This ability to offer real-time insight into brainwave activity during targeted stimulation presents an unprecedented opportunity for advancing treatment methodologies.
The Path Forward: Broader Applications and Future Research
Initial testing of the ECoG sensor on living rodents has yielded promising results, indicating its capacity for precise brainwave measurement and seizure control. The research team plans to build upon this foundation by creating a high-density electrode array capable of delivering even more detailed data analytics once it clears clinical trials. As noted by Son, expansion of electrode channels will significantly enhance the resolution, fostering enhanced diagnostic capabilities for epilepsy and potentially other neurological disorders.
The implications of this research extend beyond personalized epilepsy treatment; the ECoG sensor’s adaptability and precision may facilitate groundbreaking developments in the treatment of various brain-related conditions, and even usher in advancements in the world of prosthetics. Thus, the future of non-invasive neurological treatment appears exceedingly promising, all stemming from innovative sensor technology that could transform our understanding and management of brain disorders for years to come.