Bipolar membranes have long been recognized as a crucial component in various energy technologies, such as electrolyzers and hydrogen fuel cells. Despite their widespread use, the underlying principles governing the functionality of bipolar membranes have remained somewhat elusive. Recently, researchers at the Fritz-Haber Institute of the Max Planck Society embarked on a study to delve deep into the water dissociation and ion solvation kinetics at the interface of these membranes. Their findings, published in Nature Energy, shed new light on the fundamental workings of bipolar membranes and hold promise for the future design and optimization of these materials.
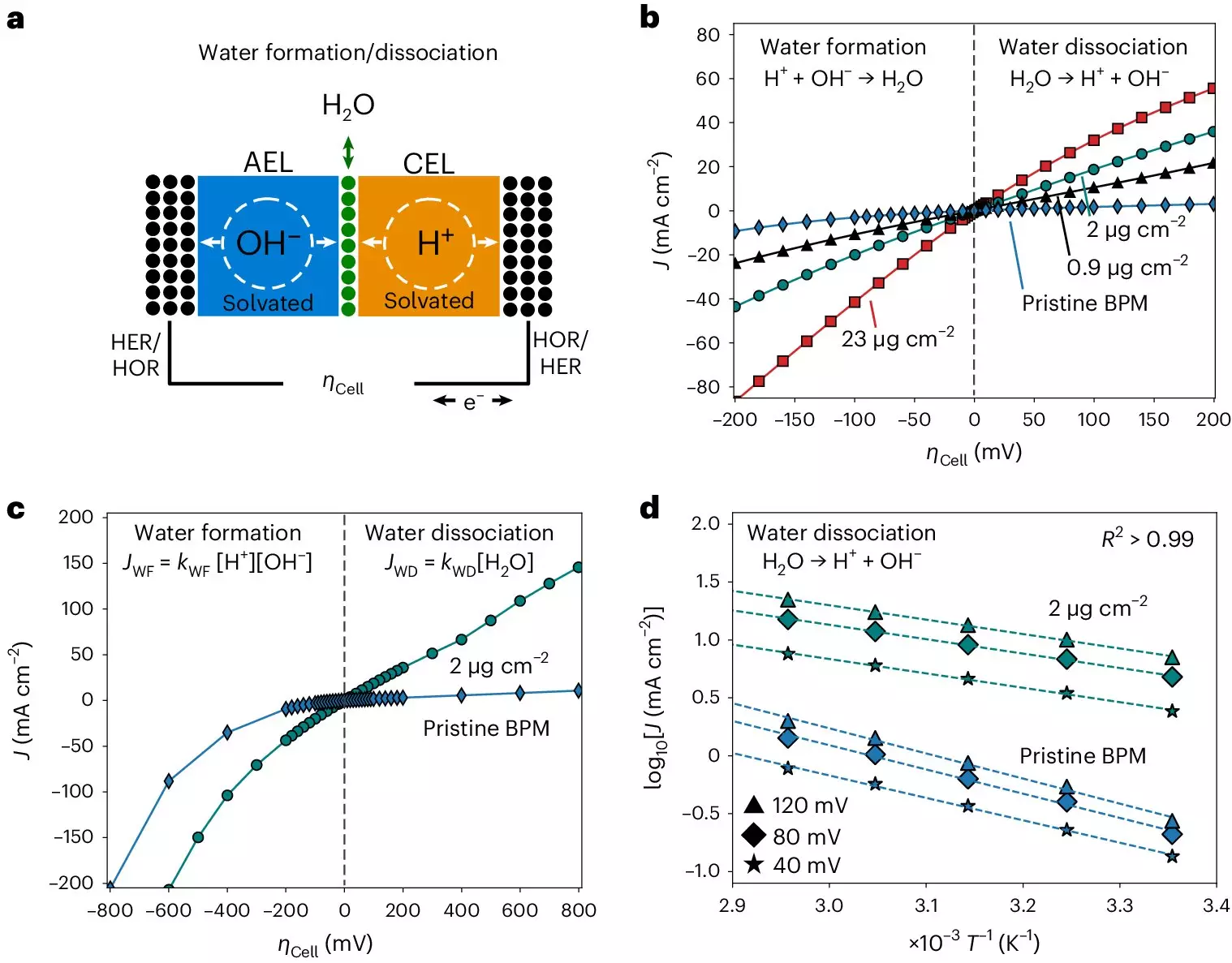
In the quest to unravel the mysteries surrounding bipolar membranes, Carlos Gomez Rodellar, a Ph.D. student at the Interface Science Department, faced numerous research challenges. One of the primary hurdles was the development of a system that could accurately measure the kinetics of bipolar membranes while minimizing interferences from electrolyte ions. This required the implementation of a setup that applied consistent physical pressure on the membrane electrode assembly, complete with metal oxide catalysts within the bipolar junction. Additionally, the system needed to allow for precise control over temperature and gas humidity to enable detailed Arrhenius analysis. Through meticulous experimentation and careful design, the research team was able to overcome these obstacles and collect valuable data on the activation entropy and enthalpy within the bipolar junction.
The measurements obtained by the researchers revealed several critical insights into the functioning of bipolar membranes. Most notably, they uncovered bias-dependent relationships between activation entropy and enthalpy, suggesting a correlation with the dispersion of interfacial capacitance. Furthermore, the team observed distinctive solvation kinetics within bipolar membranes that appeared to be driven by entropic changes in the interfacial electrolyte, rather than the chemical composition of the catalysts utilized. These findings have significant implications for enhancing the performance of bipolar membranes in various applications, including electrodialysis, CO2 electrolyzers, and hydrogen fuel cells.
The study conducted by the research group at the Fritz-Haber Institute not only deepened our understanding of bipolar membranes but also paved the way for future advancements in the field of electrochemistry. By elucidating the role of entropic changes at liquid-solid interfaces, the researchers have opened up new possibilities for designing innovative electrocatalysts that can drive specific chemical reactions, such as the production of green hydrogen from alkaline electrolytes. Moreover, the insights gained from this study could revolutionize the development of bipolar membranes for a wide range of applications, fueling advancements in fuel cell technology and electrodialysis systems.
The groundbreaking research carried out by the team at the Fritz-Haber Institute represents a significant step forward in our quest to unlock the full potential of bipolar membranes. By bridging the gap between theory and experimentation, the researchers have shed light on the intricate workings of these essential materials, offering new pathways for innovation and discovery in the realm of energy technologies. As we look towards the future, continued exploration of the fundamental principles governing bipolar membranes promises to yield transformative advancements that could shape the landscape of sustainable energy production for years to come.