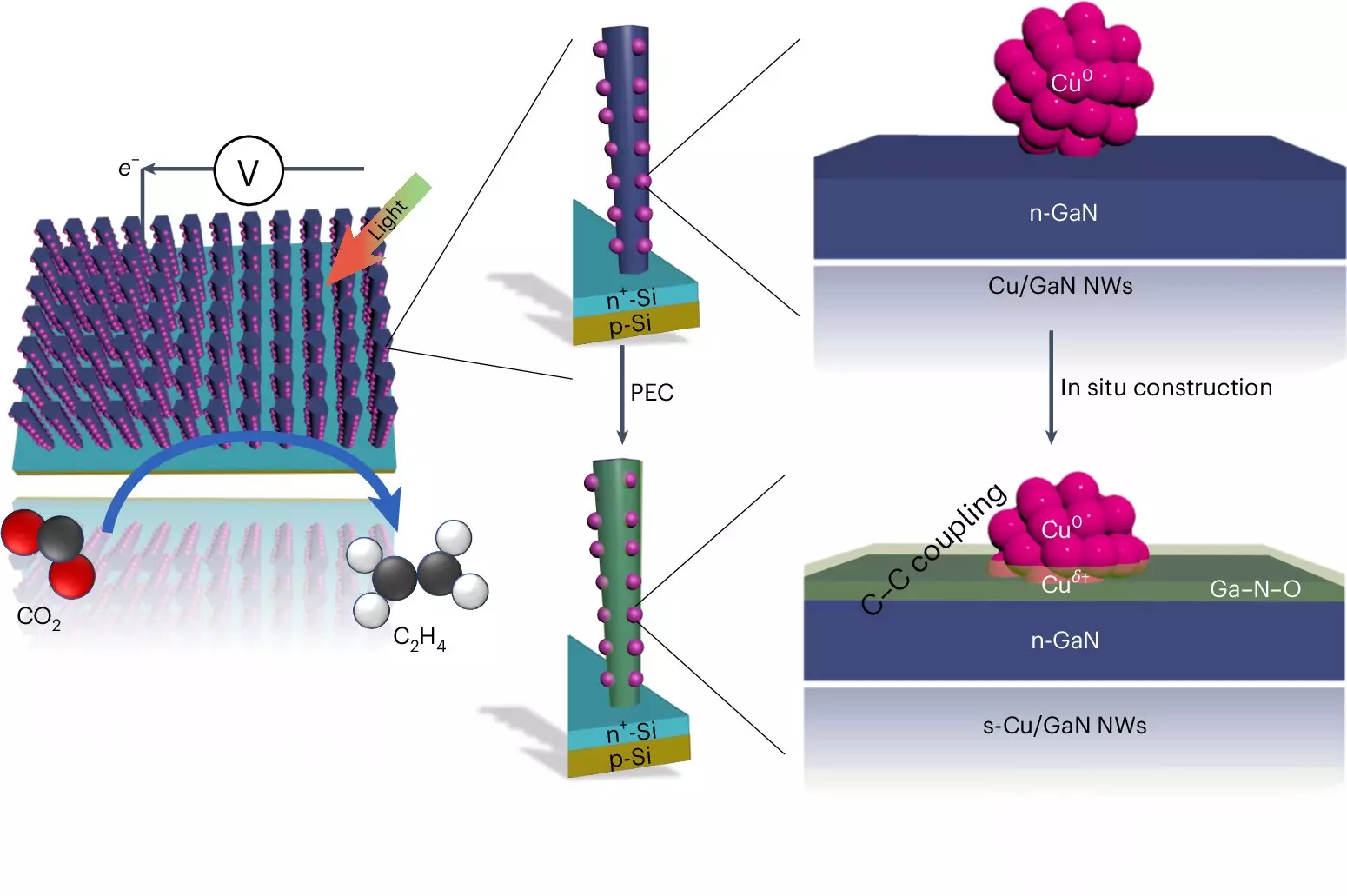
The increasing urgency to address climate change has spurred innovative research into technologies that repurpose carbon dioxide (CO2) into valuable resources. At the forefront of this movement is an artificial photosynthesis system developed by a research team at the University of Michigan. This cutting-edge system represents a crucial advancement in the quest to convert atmospheric carbon into sustainable fuels, specifically focusing on the production of ethylene, a fundamental hydrocarbon used predominantly in the plastics industry.
Ethylene is recognized as the most widely produced organic compound globally; however, its traditional manufacturing process involves the use of fossil fuels, which contributes significantly to greenhouse gas emissions. By innovatively harnessing CO2, the University of Michigan’s system positions itself as a cleaner alternative, suggesting that captured atmospheric CO2 can be redirected to create essential materials without exacerbating environmental issues. Professor Zetian Mi highlights the remarkable efficiency of their system, which operates at five to six times the performance levels compared to other existing technologies. This advancement marks a significant stride toward integrating sustainability within the plastics sector and beyond.
At the heart of this technology lies a unique mechanism involving gallium nitride (GaN) nanowires and copper clusters. The nanowires serve as the platform for chemical reactions, facilitating the absorption of solar energy, which effectively splits water into hydrogen and oxygen. When exposed to sunlight, each nanowire—measuring just 50 nanometers in width—interacts with a copper catalyst that manages the complex reaction of converting CO2 into ethylene. This process is particularly intricate; it requires exceptional coordination between light-driven water splitting and the catalytic properties of copper clusters.
The electrical energy generated during this photochemical reaction is pivotal. It frees up electrons, which then assist in reducing CO2 to carbon monoxide, a necessary precursor to ethylene synthesis. The synergistic relationship between the GaN nanowires and the copper catalyst not only enhances the reaction’s efficiency but also allows for a self-healing mechanism by incorporating oxygen from the reaction’s by-products. Such innovative design showcases the potential for improving catalyst longevity, creating a more stable and efficient energy conversion system.
A critical aspect of the efficacy of the University of Michigan’s artificial photosynthesis system is its exceptional stability over extended periods. While competing systems experience rapid degradation, often lasting mere hours, the Michigan team’s device has demonstrated remarkable persistence, operating for over 116 hours without deterioration. Furthermore, similar setups have been run successfully for 3,000 hours. This milestone is essential as it aligns with the goal of developing resilient technologies capable of sustaining long-term operations.
The efficiency achieved—where 61% of the generated electrons contribute to ethylene production—is another remarkable achievement. It starkly contrasts with alternative methods, such as those utilizing a silver and copper catalyst which, although effective, demanded carbon-based fluids and exhibited a limited operational window. The successful integration of oxygen enrichment within the gallium nitride framework emerges not just as an enhancement but as a potential game changer for catalyst development, setting a precedent for future innovations.
As the research team aims to further enhance this technology, future work promises to delve into the synthesis of more complex hydrocarbons, potentially paving the way for liquid fuels like propanol. These advancements could ultimately redefine energy sources for transportation, rendering them more environmentally friendly and sustainable. Professor Mi envisions an ecosystem where carbon emissions are rechannelled into valuable products, thereby curbing reliance on fossil fuels and minimizing environmental impact.
This groundbreaking research exemplifies how interdisciplinary approaches can yield innovative solutions to ongoing global challenges. By shifting towards systems that convert CO2 into critical materials rather than allowing it to escape into the atmosphere, scientists at the University of Michigan are paving the way for a more sustainable future.
The artificial photosynthesis system developed at the University of Michigan stands as a promising testament to the potential of innovative technologies in tackling climate change. By efficiently repurposing CO2 into ethylene, this research has opened avenues for sustainable fuel production and reinforced the viability of carbon capture strategies. As the exploration of longer carbon chains initiates, the dream of transforming our environmental liabilities into sustainable energy assets becomes increasingly attainable. This revolutionary approach aspires to shape a circular economy where carbon is not merely a waste product but a valued resource.