Fuel cells have long been hailed as a revolutionary energy-conversion solution that could potentially power a wide range of technologies without contributing to air pollution. However, despite their numerous advantages, the widespread adoption of fuel cells has been hindered by the reliance on expensive materials and precious metal catalysts. This limitation has sparked the interest of researchers worldwide to explore alternative designs that are more cost-effective and sustainable.
One promising solution that has emerged is the development of anion-exchange-membrane fuel cells (AEMFCs). These fuel cells are based on Earth-abundant, low-cost catalysts, making them more affordable and accessible for various applications. While previous iterations of fuel cell designs have faced challenges with the oxidation of non-precious metal catalysts, researchers at Chongqing University and Loughborough University have recently introduced a groundbreaking strategy to address this issue.
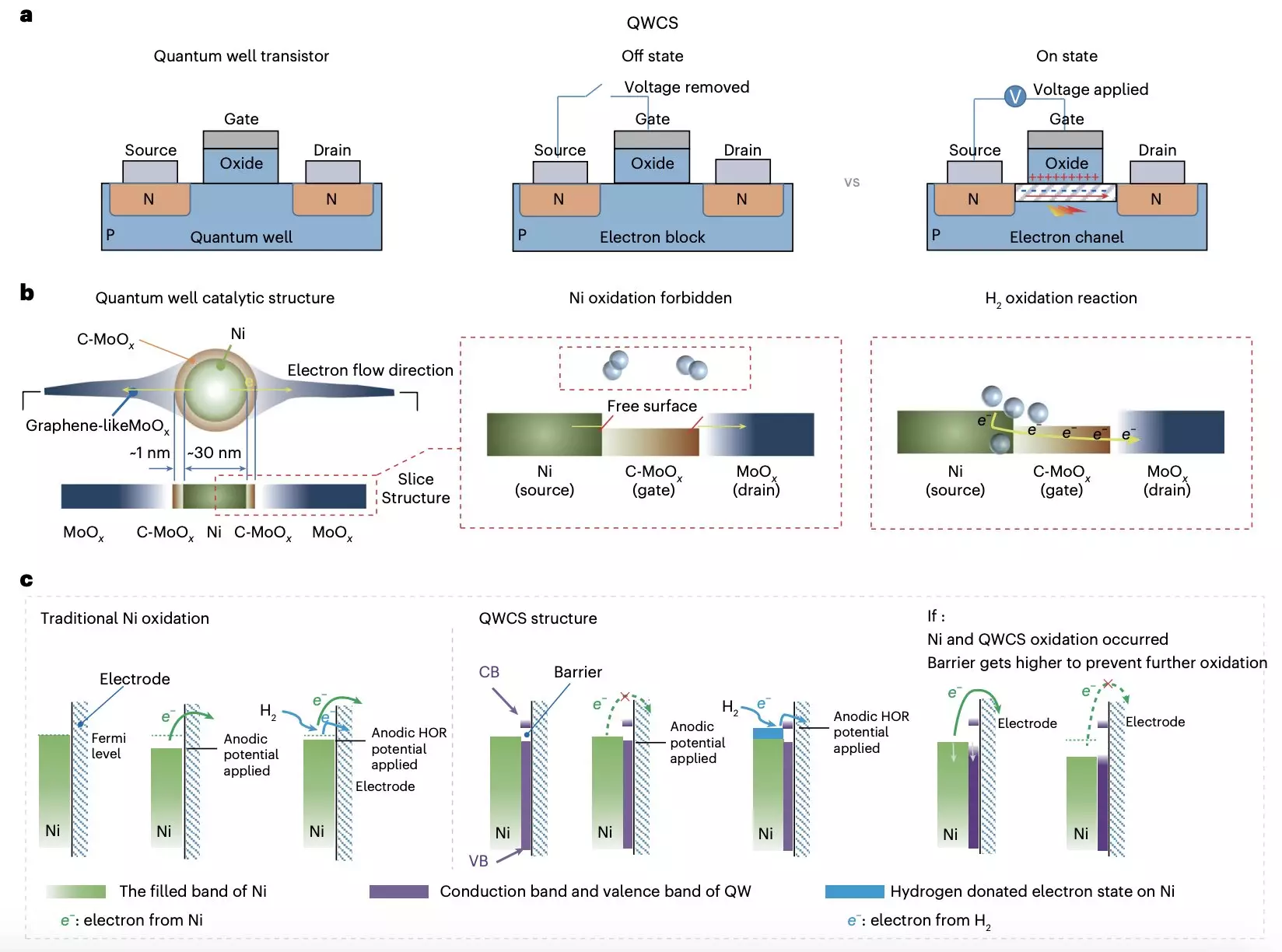
In a recent publication in Nature Energy, Yuanyuan Zhou, Wei Yuan, and their colleagues unveiled a new quantum well-like catalytic structure (QWCS) that aims to prevent the oxidation of metallic nickel electrocatalysts in AEMFCs. This innovative design involves the creation of a quantum-confined metallic nickel nanoparticles structure that can selectively transfer external electrons from the hydrogen oxidation reaction while maintaining its metallic properties. This selective electron transfer mechanism effectively protects the catalyst from electro-oxidation, ensuring the long-term stability and performance of the fuel cell.
The QWCS developed by the research team consists of nickel nanoparticles confined within a carbon-doped MoOx/MoOx heterojunction. This unique structure leverages quantum well properties to enhance catalytic activity and stability. The catalyst, known as Ni@C-MoOx, demonstrated exceptional catalytic stability during prolonged operation under harsh conditions, showcasing a high specific power density and consistent performance after repeated shutdown-start cycles.
The successful implementation of the QWCS-catalyzed AEMFC marks a significant milestone in the development of cost-effective and reliable fuel cells. By leveraging quantum confinement to protect non-precious metal catalysts from oxidation, this innovative design could pave the way for future advancements in fuel cell technology. The underlying principles of the QWCS approach have the potential to inspire the creation of new catalysts that exhibit enhanced stability and performance, further driving the evolution of sustainable energy solutions.
The introduction of the quantum well-like catalytic structure represents a major breakthrough in fuel cell technology. By addressing the limitations of traditional catalyst designs, researchers have unlocked new possibilities for the development of affordable and efficient energy-conversion solutions. As we continue to explore the potential of quantum confinement in catalyst design, we may witness a transformative shift towards a greener and more sustainable future powered by innovative fuel cells.