Lithium-metal batteries have long been hailed as the next big advancement in battery technology, with the potential to significantly surpass the energy densities of current lithium-ion batteries. However, despite their promise, lithium-metal batteries have been plagued by issues such as short lifespans and the formation of lithium dendrites. These limitations have prevented the widespread adoption of lithium-metal batteries in commercial applications.
One of the primary challenges facing lithium-metal batteries is their limited cycle life, typically around 50 cycles, compared to the approximately 1,000 cycles of commercial lithium-ion batteries. The growth of lithium dendrites, high reactivity of lithium-metal, and degradation of the electrolyte contribute to this shortened lifespan. These issues have hindered the practical applications of lithium-metal batteries and have prevented them from reaching their full potential in the market.
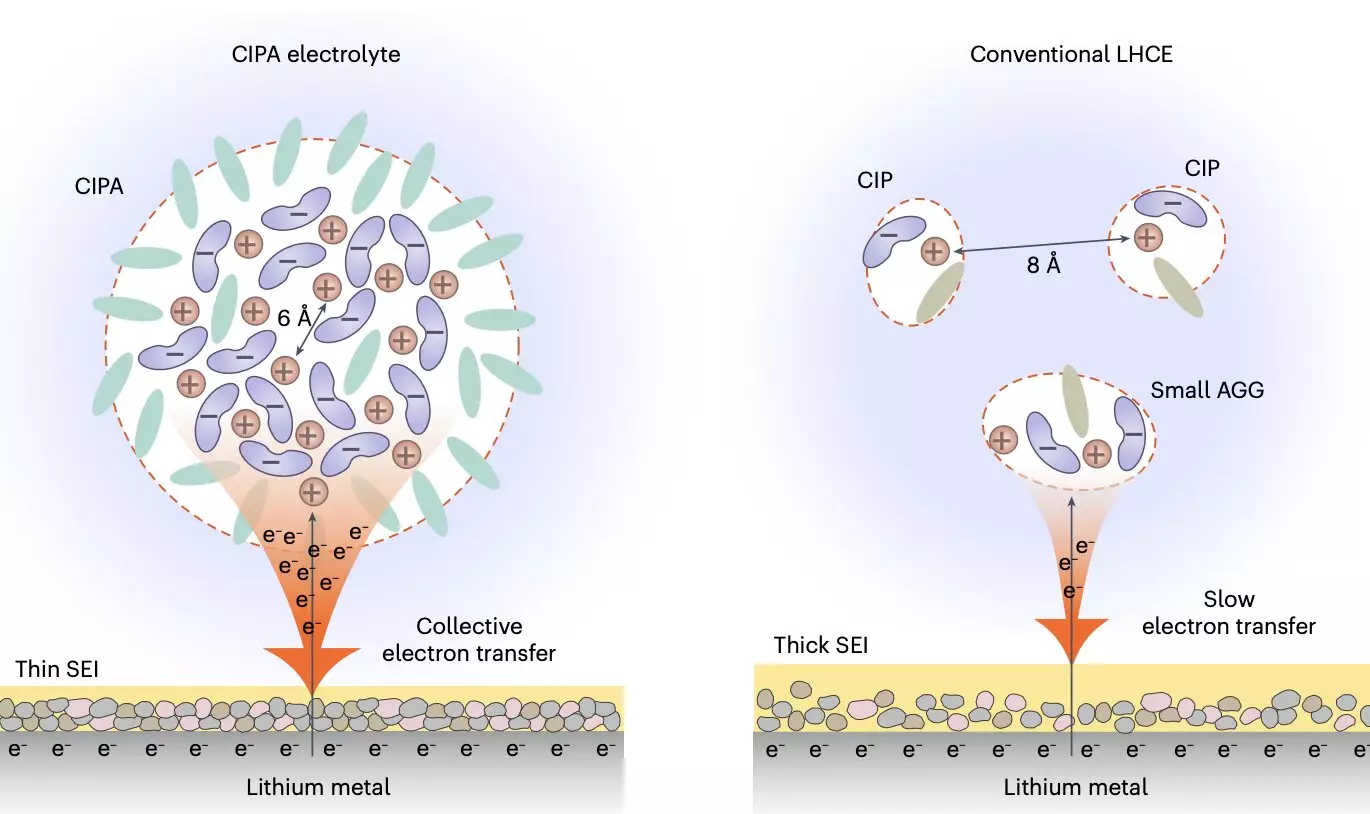
Recently, researchers at the University of Science and Technology of China introduced a new electrolyte design that could address some of the limitations of lithium-metal batteries. This electrolyte features a unique nanometer-scale solvation structure, with pairs of ions densely packed together into compact ion-pair aggregates (CIPA). The goal of this new electrolyte design is to stabilize the anode-electrolyte and cathode-electrolyte interfaces in lithium-metal battery cells, thereby extending the lifespan of the batteries.
By tuning the solvation structure of the electrolyte at the mesoscopic level, the researchers were able to create a new class of electrolytes that exhibit promising results for lithium-metal batteries. The electrolyte’s unique design focuses on ion pairs and their interaction, leading to the formation of CIPA structures that promote a thin and stable solid electrolyte interface (SEI) on the lithium-metal anode. This SEI suppresses electrolyte degradation and enables homogeneous lithium deposition, reducing the risk of dendrite formation.
The introduction of this new electrolyte design opens up new possibilities for the future of lithium-metal batteries. Initial tests of a 500 Wh/kg lithium-metal pouch cell using this electrolyte showed promising results, with the cell retaining 91% of its energy after 130 cycles. The potential for further improving the cycle life of these batteries to over 1,000 cycles is being explored, along with the goal of achieving even higher energy densities, such as ≥ 600 Wh/kg with 100-200 cycles.
The development of a new electrolyte design for lithium-metal batteries represents a significant step forward in overcoming the limitations of current battery technology. By focusing on the solvation structure of the electrolyte and its interaction with ion pairs, researchers have been able to create a more stable and long-lasting energy storage solution. The future of lithium-metal batteries looks bright, with the potential to revolutionize the electric vehicle industry and other energy storage applications.